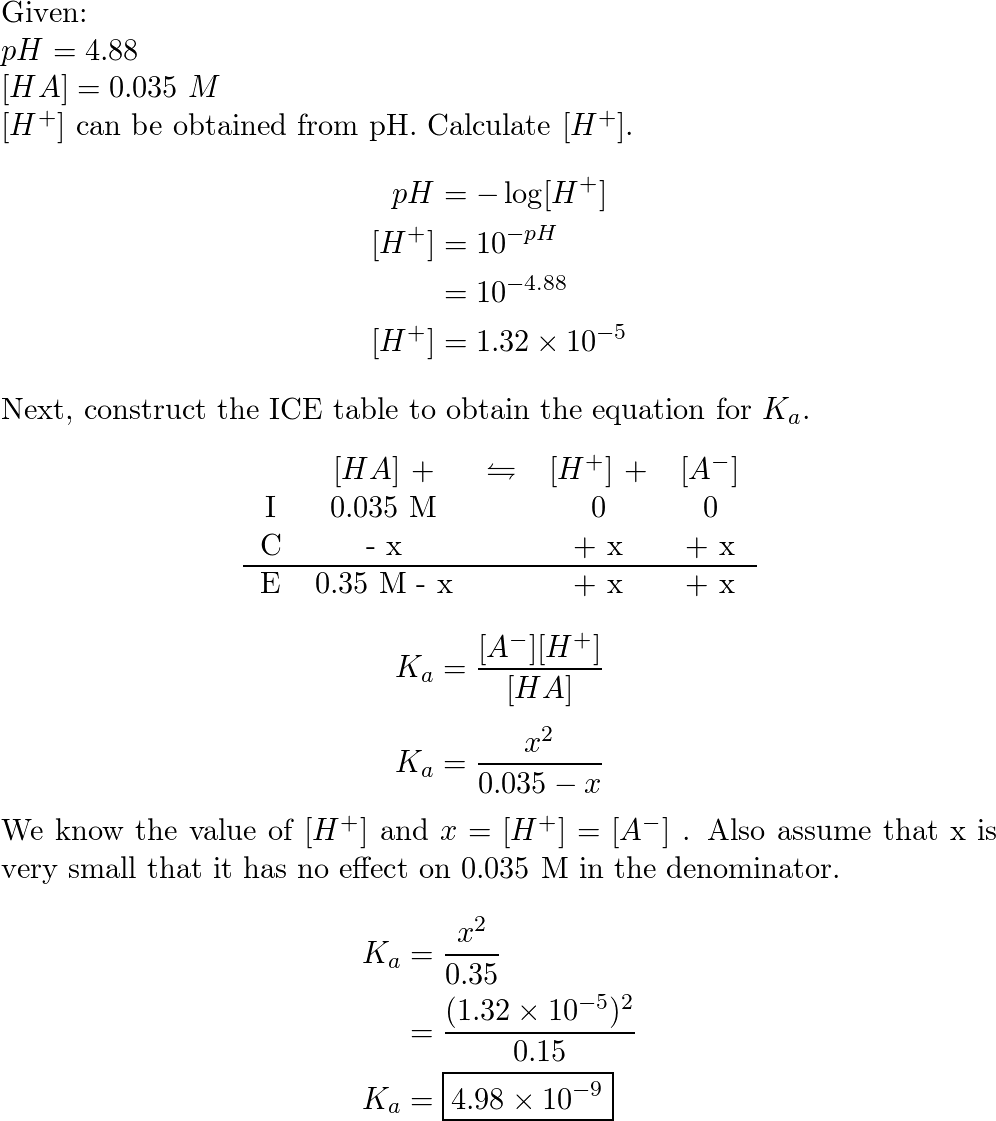HHELIKOPTER/HELIKOPTER - Magyarország legnagyobb helikopteres repüléssel foglalkozó oldala - Helikopter képadatbázis

Ka Sharak - Ka Sharak Khasi Daily Devotional 17th February: KA KTIEN U BLEI Salm 119:105 "Ka ktien jong Me ka long ka sharak ia ki kjat jong nga, bad ka jingshai

HHELIKOPTER/HELIKOPTER - Magyarország legnagyobb helikopteres repüléssel foglalkozó oldala - Helikopter képadatbázis

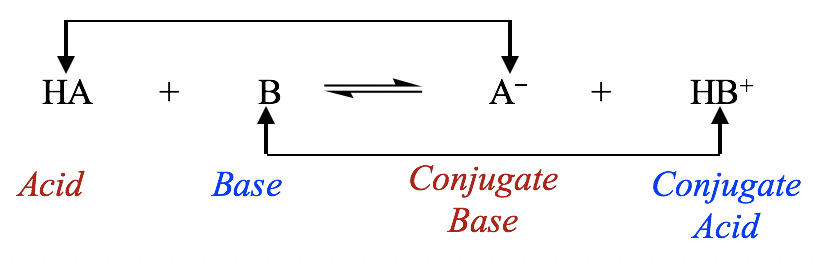
The weak acid, HA has a Ka of 1.00 × 10^-5 . If 0.1 mol of this acid is dissolved in one litre of water, the percentage of acid dissociated at equilibrium is closet to:
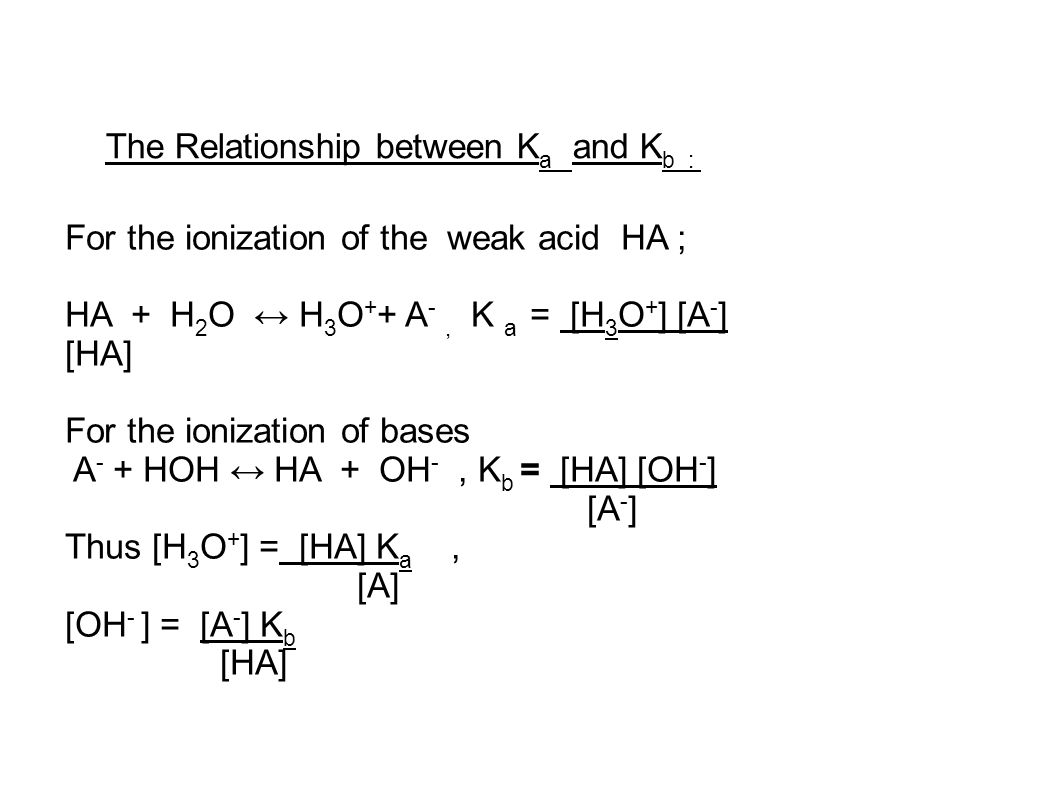
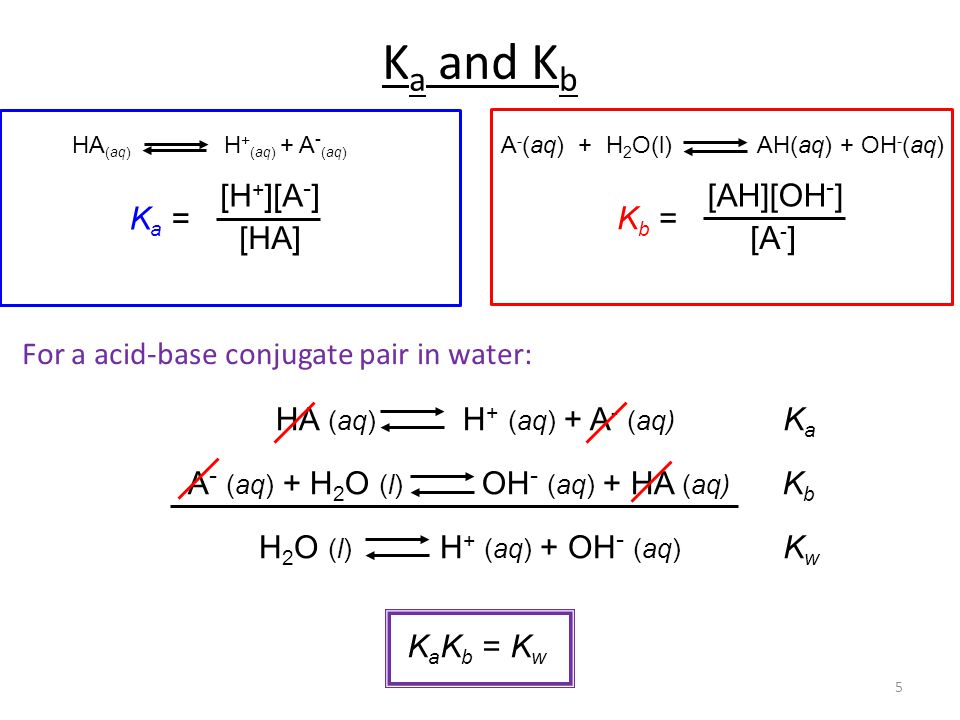
The Relationship between K a and K b : For the ionization of the weak acid HA ; HA + H 2 O ↔ H 3 O + + A -, K