Calculate pH of the following mixtures. Given that Ka = 1.8 × 10^–5 and Kb = 1.8 × 10^–5: - Sarthaks eConnect | Largest Online Education Community
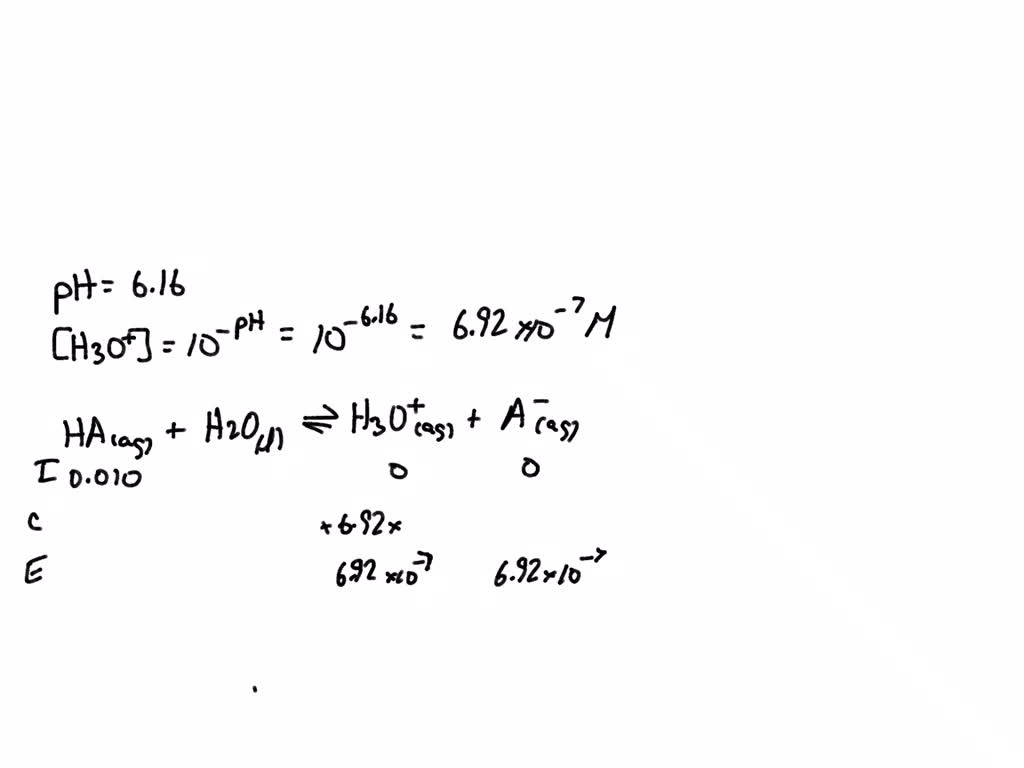
SOLVED: The pH of an acid solution is 6.16. Calculate the Ka for the monoprotic acid. The initial acid concentration is 0.010 M. Ka = ? x 10^? Enter the answer in scientific notation


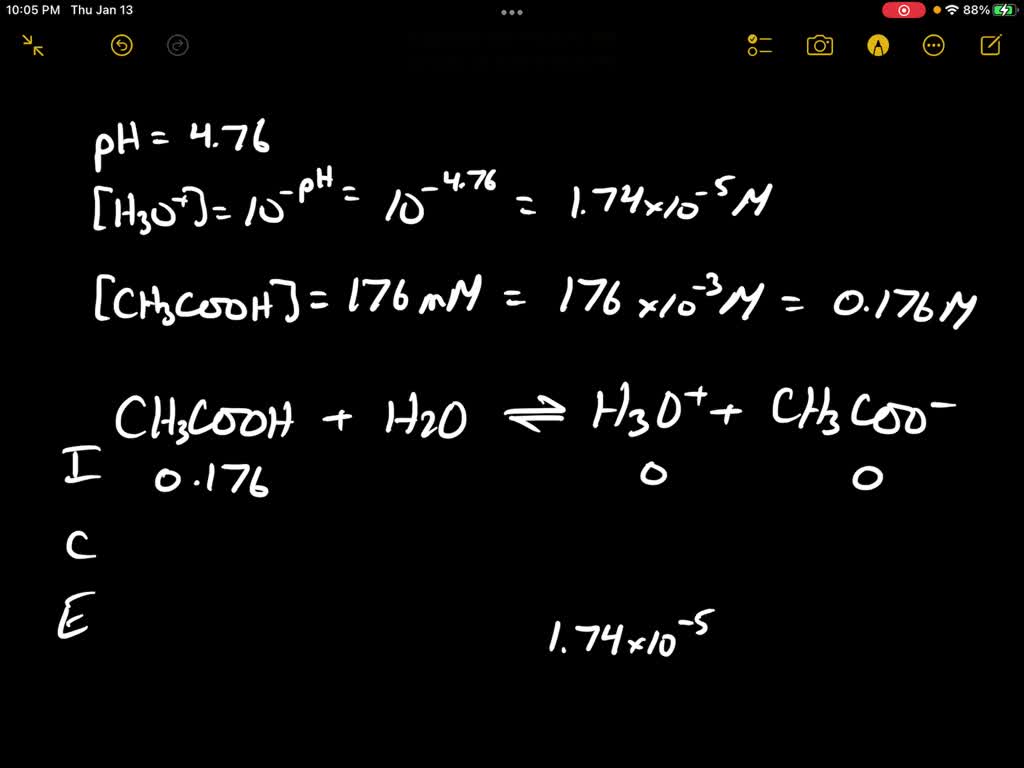
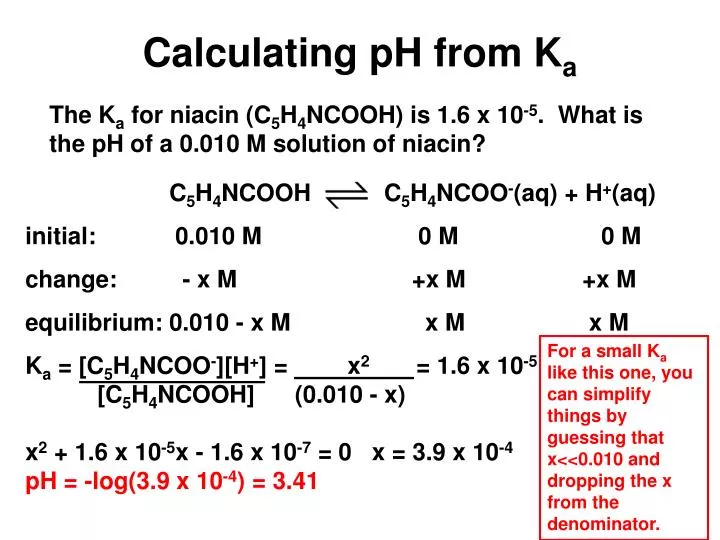

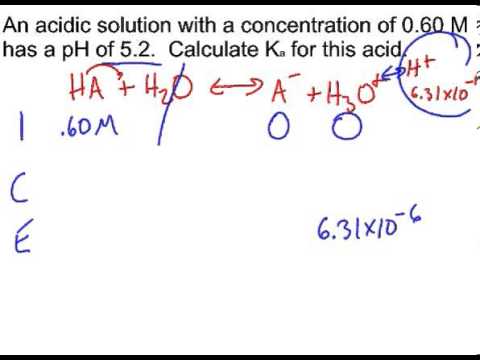
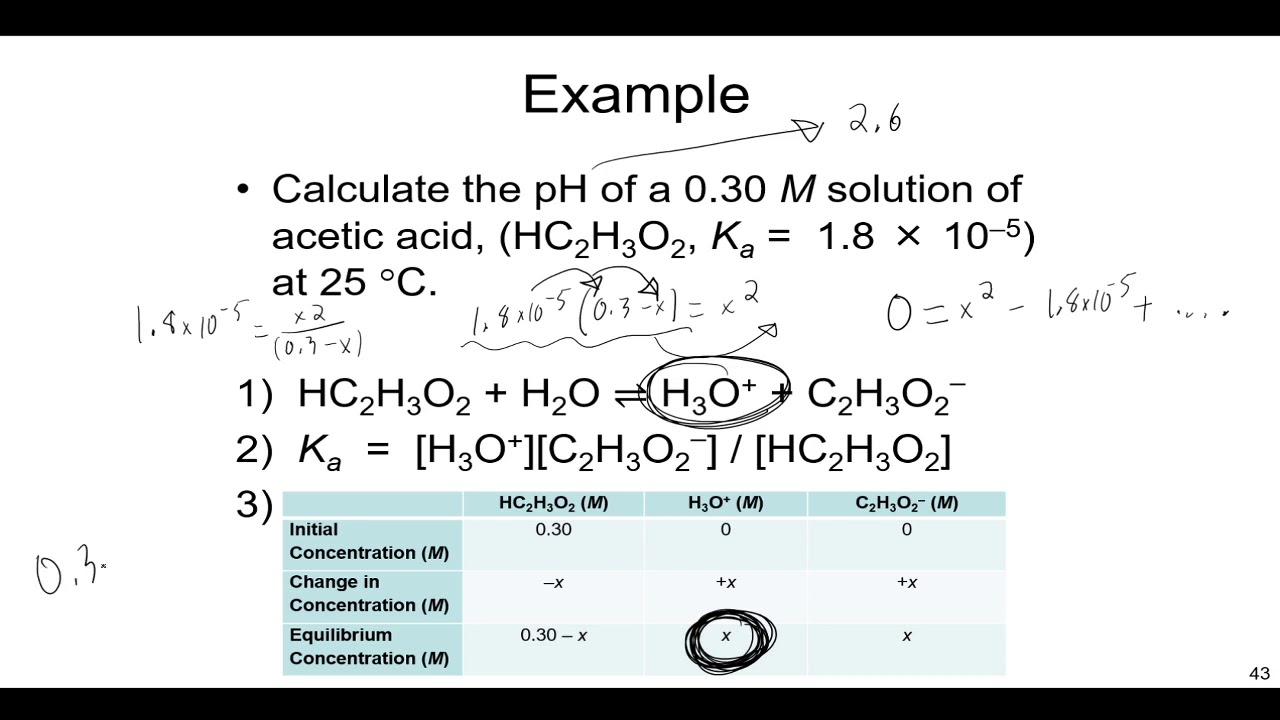
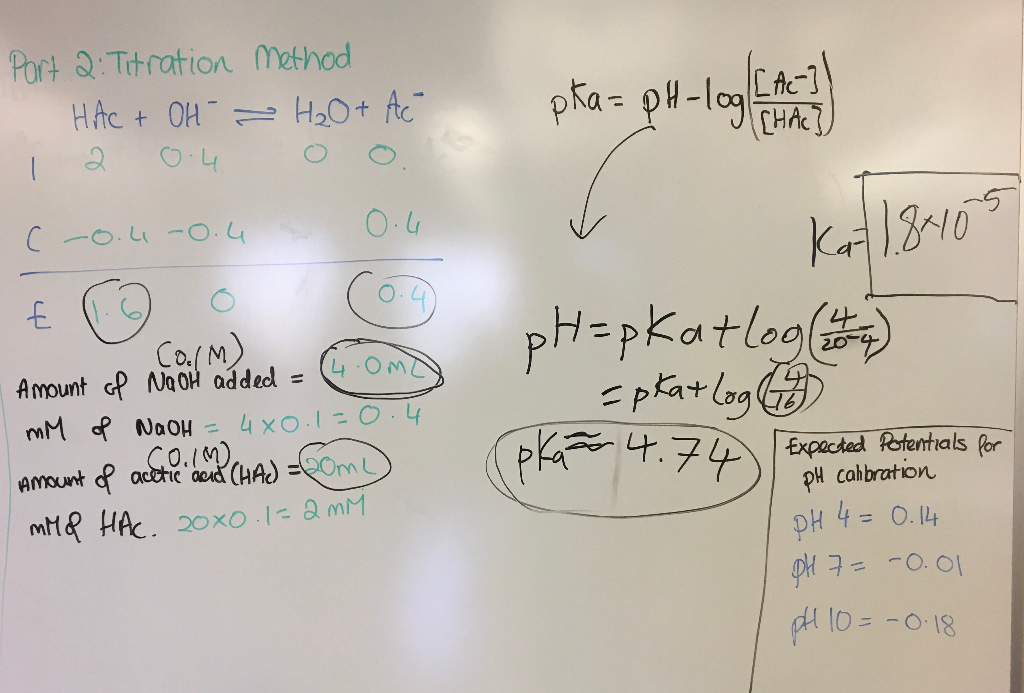


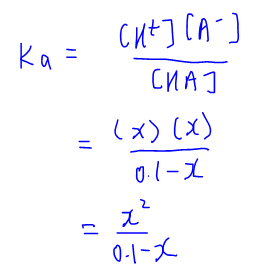

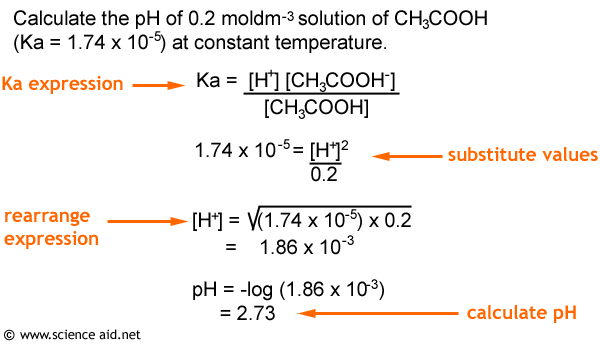



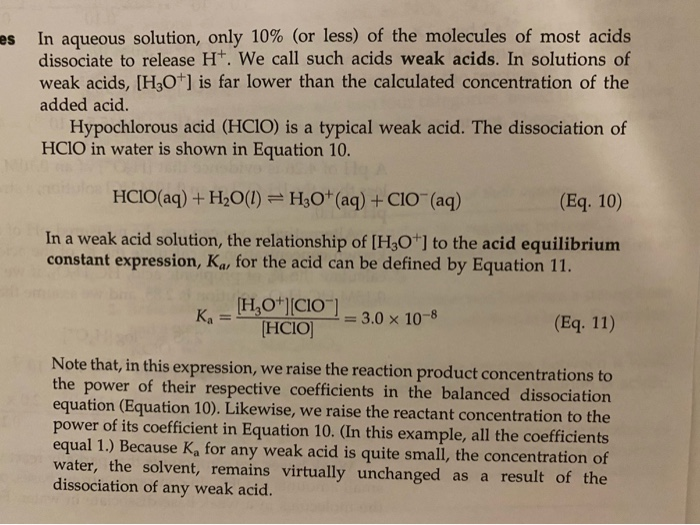


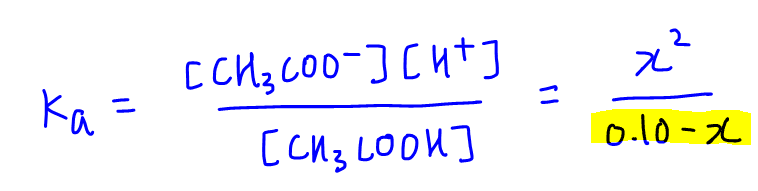
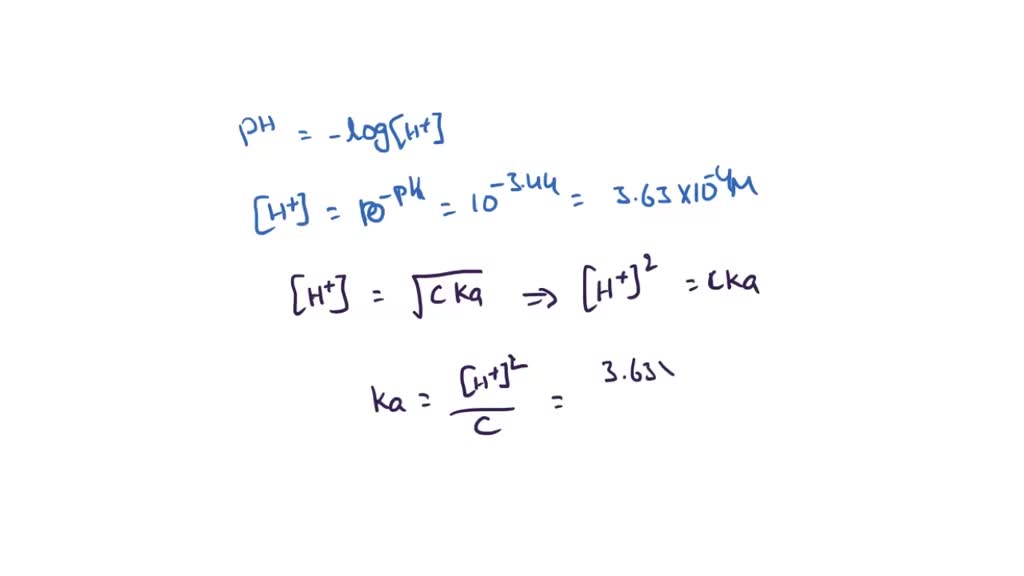


![Calculating [H+] and pH from Ka Calculating [H+] and pH from Ka](https://www.mi.mun.ca/users/pfisher/chemistry1011_134/img013.gif)