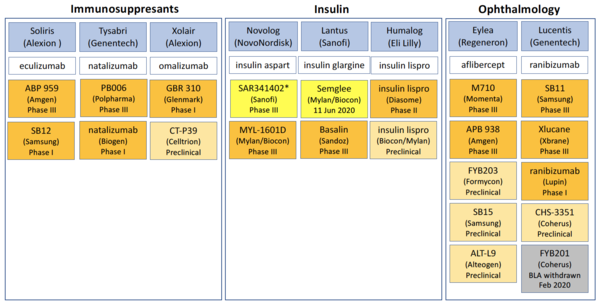
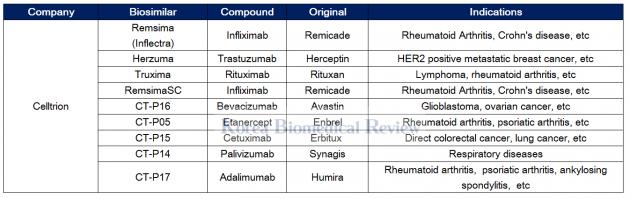
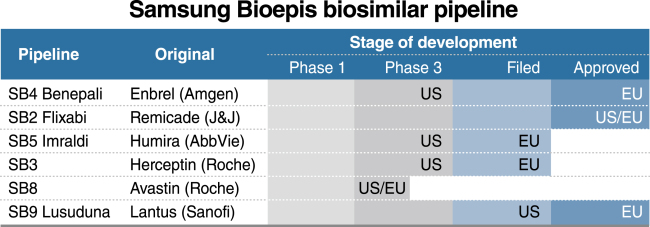
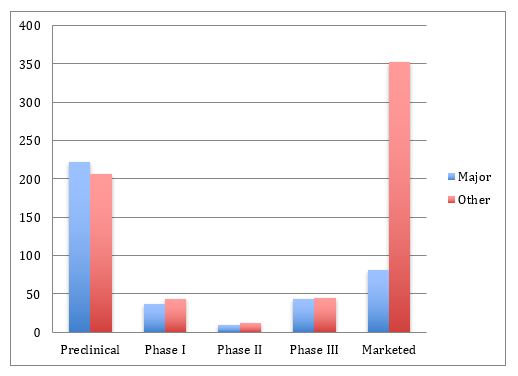
How the U.S. Compares to Europe on Biosimilar Approvals and Products In the Pipeline | Biosimilars Law Bulletin

Samsung Bioepis Enters into Commercialization Agreement for Next-Generation Biosimilar Candidates | Business Wire

Samsung Bioepis' Biologics License Application for SB2 Infliximab Biosimilar Accepted by U.S. Food and Drug Administration | Business Wire